Grant number: 2016/21/B/NZ7/01081
Principal Investigator: Małgorzata Brindell
Source of funding: National Science Center Poland
According to the World Health Organization (WHO), cancer is one of the leading causes of morbidity and mortality worldwide. The biggest problem in the treatment of cancer is the development of metastases. It is estimated that over 90% of cancer-related deaths are associated with metastases. The metastasis process has multiple stages, and each of these may be the target of therapeutic intervention. Despite its enormous complexity, metastasis is not unique, but it occurs in almost all types of cancer, with the only difference being the stage in which the process is initiated. To adapt anticancer therapy to this phenomenon, in addition to cytotoxic properties, a potential therapeutic compound should inhibit the formation of metastases, possibly at many stages of the process. Out of all the compounds we have tested so far, one of them has shown very promising properties, [Ru(dip)2(bpy-SC)]2+(shown in Figure).
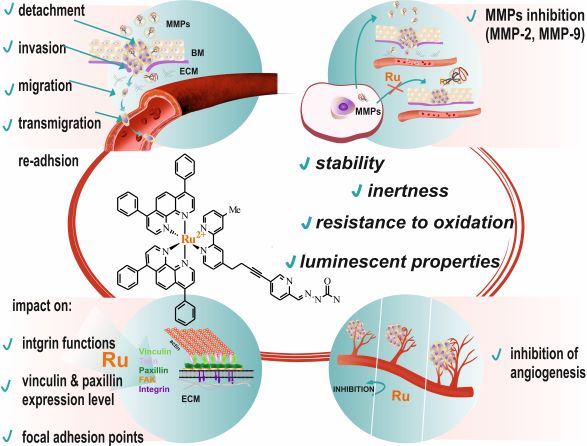 The tested compound showed cytotoxicity for various types of cancer cells at concentrations of several micrograms per milliliter. Furthermore, when administered in non-toxic doses, it modified the adhesive properties of the cells, which resulted in inhibiting the migration and invasion of cancer cells and in influencing the angiogenesis process of endothelial cells, among others. One of the more significant achievements of this project was the demonstration that the test compound does not only have a single molecular target but controls the metastasis process at its various stages.
The tested compound showed cytotoxicity for various types of cancer cells at concentrations of several micrograms per milliliter. Furthermore, when administered in non-toxic doses, it modified the adhesive properties of the cells, which resulted in inhibiting the migration and invasion of cancer cells and in influencing the angiogenesis process of endothelial cells, among others. One of the more significant achievements of this project was the demonstration that the test compound does not only have a single molecular target but controls the metastasis process at its various stages.
We believe that the results of this project will bring new solutions beyond the typical approach in metal drug design and also will draw researchers' attention to the fact that metastasis is the most important target of potential drugs. The combination of a cytotoxic effect with an antimetastatic one seems to be the proper approach in drug design, and the use of metal complexes as metastasis inhibitors may open a new chapter in medical chemistry.
I. Gurgul, O. Mazuryk, M. Łomzik, Ph. C. Gros, D. Rutkowska-Żbik, M. Brindell
Metallomics 2020, 12, 784-793
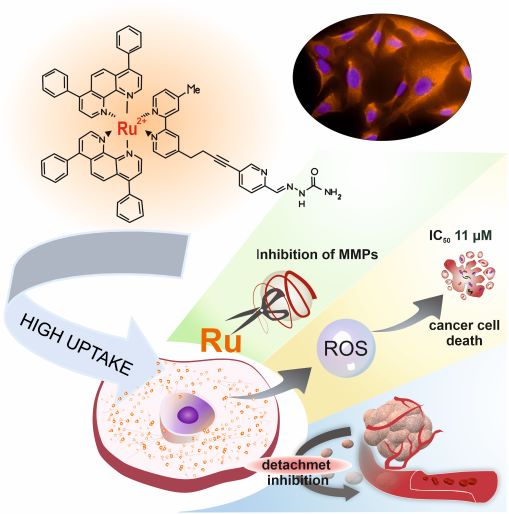
The well-documented cytotoxic activity of coordinatively saturated and substitutionally inert polypyridyl Ru(II) complexes substantiates to their high potency as antiproliferative agents against primary tumors. However, the primary cause of cancer morbidity and mortality is the occurrence of metastasis. Therefore, we focus on evaluating the effect of Ru(II) polypyridyl complexes bearing 2,2’-bipyridine substituted by a semicarbazone 2-formylopyridine moiety on cellular features important in the development of metastasis. Some of the studied complexes strengthen cells’ adherent properties as well as inhibit the activity of metalloproteinases (MMPs) in vitro, which is relevant in anti-metastatic treatment. The in vitro studies revealed that the induced alteration of tumor cells’ adhesion properties correlated with the high cytotoxic effect exerted by the complexes and their excellent cellular uptake. It was also proved that both complexes directly inhibit MMP2 and MMP9 enzyme activities, which are essential for the development of tumor metastasis. The results of this study indicate that the biological properties of polypyridyl Ru(II) complexes extend beyond the standard cytotoxic activity and represent an important step towards designing new anti-metastatic agents. We anticipate that our findings will draw the attention of scientists to the unrecognized, underrated properties of polypyridyl Ru(II) complexes towards their application as antimetastatic agents.
P. Gajda-Morszewski, I. Gurgul, E. Janczy-Cempa, O. Mazuryk, M. Łomzik, M. Brindell
Pharmaceuticals 2021, 14, 1014
Inhibiting metastasis at the very beginning is essential for successful anticancer therapy. Cell detachment from the primary tumor followed by invasion is considered the early phase of metastasis. Among the studies series of Ru(II) polypyridyl complexes three of them namely [Ru(dip)2(bpy-SC)]2+, [Ru(dip)2(dpb)]2+ and [Ru(dip)3]2+ (dip: 4,7-diphenyl-1,10-phenanthroline; bpy-SC: bipyridyne derivative bearing a semicarbazone 2-formylopyridine moiety; dpb: quinoxaline derivatives) demonstrated high activity in inhibiting of cell detachment along with quite efficient inhibition of MMP-2 and MMP-9. More detailed studies for [Ru(dip)2(bpy-SC)]2+ showed its preferential accumulation in the cytoskeleton, where also intracellular MMP-9 was localized to a greater extent, pointing to this enzyme as one of the targets for this compound. Taken together, our results indicate the possibility of a reduction of metastatic cells escaping from the primary lesion to the surrounding tissue by prevention of their detachment and by influencing the activity of MMP-2 and MMP-9. The studied compounds seem to be good candidates for their application as antimetastatic agents.
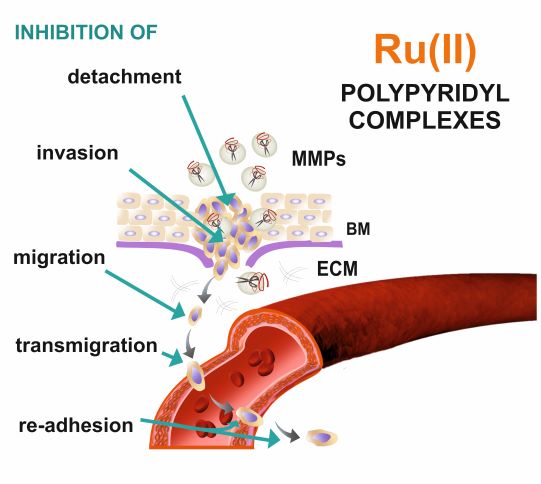 Moving Ru polypyridyl complexes beyond cytotoxic activity towards metastasis inhibition
Moving Ru polypyridyl complexes beyond cytotoxic activity towards metastasis inhibition
M. Brindell, I. Gurgul, E. Janczy-Cempa, P. Gajda-Morszewski, O. Mazuryk
J. Inorg. Biochem. 2022, 226, 111652
In recent years, Ru polypyridyl complexes have been intensively studied for their anticancer activity. The vast majority of research focuses on assessing their cytotoxic activity, as well as targeting cancer cells with them. Since the formation of metastases poses a greater risk than primary tumors, scientists recently began evaluating these compounds as potential metastasis inhibitors. This review highlights the latest achievements in this field with particular attention to the identification of the target proteins responsible for such activity. Cell migration, invasion, and adhesion are key components of metastasis, therefore understanding how they are affected by Ru polypyridyl complexes is of great importance.
I. Gurgul, E. Janczy-Cempa, O. Mazuryk, M. Lekka, M. Łomzik, F.Suzenet, P. C. Gross, M. Brindell
Journal of Medicinal Chemistry 2022, 65, 10459-10470
We have reported that a family of Ru complexes can regulate the cell adhesion properties important in metastasis development. The low sub-toxic doses of the studied compounds greatly affected cancer cells by inhibiting cell detachment, migration, invasion, transmigration, and re-adhesion, as well as increasing cell elasticity. The insight into the molecular bases of the observed cell functional changes revealed that the studied compounds influence the integrin functionality and expression of the focal adhesion components vinculin and paxillin, resulting in an increased number of focal adhesion contacts. Taken together, it seems that studied Ru compounds can act simultaneously on several targets in the metastatic cascade which make them interesting candidates for their application as efficient antimetastatic agents. Applying hypoxic conditions, often encounter in solid tumors, did not change the cytotoxicity of the studied compounds in a significant manner, but their influence on cell adhesion and mobility was smaller.
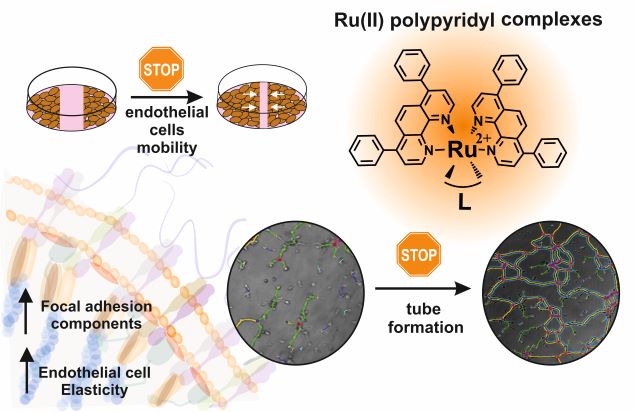 Impact of polypyridyl Ru complexes on angiogenesis - contribution to their antimetastatic activity
Impact of polypyridyl Ru complexes on angiogenesis - contribution to their antimetastatic activity
I. Gurgul, O. Mazuryk, K. Stachyra, R. Olszanecka, M. Lekka, M. Łomzik, F.Suzenet, P. C. Gross, M. Brindell
Int. J. Mol. Sci. 2022, 23, 7708
We have reported that a family of Ru complexes can deregulate the functionality of endothelial cells which may lead to the inhibition of angiogenesis. The low sub-toxic doses of the studied compounds greatly affected the endothelial cell migration and pseudovessels formation. Functional changes in endothelial cells might arise from the impact of the studied compounds on cell elasticity and expression of proteins (vinculin and paxillin) involved in focal adhesions. Furthermore, molecular studies showed that complexes modulate the expression of cell adhesion molecules, which has been suggested to be one of the factors that mediate the activation of angiogenesis.

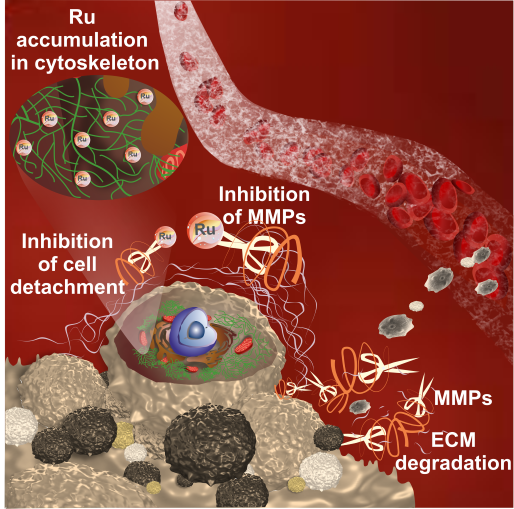 Inhibition of matrix metalloproteinases and cancer cell detachment by Ru(II) polypyridyl complexes containing 4,7-diphenyl-1,10-phenanthroline ligands - new candidates for antimetastatic agents
Inhibition of matrix metalloproteinases and cancer cell detachment by Ru(II) polypyridyl complexes containing 4,7-diphenyl-1,10-phenanthroline ligands - new candidates for antimetastatic agents 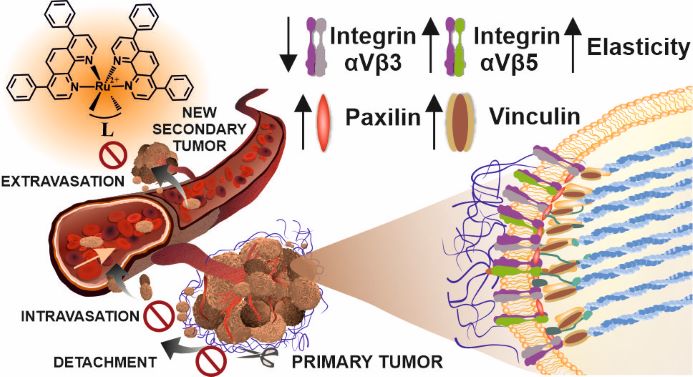 Inhibition of metastasis by polypyridyl Ru(II) complexes through modification of cancer cell adhesion – in vitro functional and molecular studies
Inhibition of metastasis by polypyridyl Ru(II) complexes through modification of cancer cell adhesion – in vitro functional and molecular studies